Students must start practicing the questions from CBSE Sample Papers for Class 9 Science with Solutions Set 8 are designed as per the revised syllabus.
CBSE Sample Papers for Class 9 Science Set 8 with Solutions
Time : 3 Hrs
Max. Marks: 80
General Instructions
- This question paper consists of 39 questions in 3 sections. Section A is Biology, Section B is Chemistry and Section C is Physics.
- All questions are compulsory. However, an internal choice is provided in some questions.
- A student is expected to attempt only one of these questions.
Section – A
Question 1.
In which group do all items contribute both nutrients and beneficial microbes to the soil? [1]
(a) Chemical fertilisers, urea, compost
(b) Cow dung, compost, green manure
(c) Urea, farmyard manure, neem cake
(d) Chemical fertilisers, neem leaves, urea
Answer:
(b) Cow dung, compost, green manure
Explanation:
Cow dung, compost, green manure, they are the examples of organic manures that improve soil texture and add beneficial microbes that help in maintaining soil fertility.
Question 2.
Which tissue type is involved in both voluntary and involuntary movement in animals? [1J
(a) Connective tissue
(b) Nervous tissue
(c) Muscular tissue
(d) Epithelial tissue
Answer:
(c) Muscular tissue
Explanation:
Muscular tissue is involved in both voluntary and involuntary movement in animals and it is responsible for movement in animals.
Question 3.
Which of the following options correctly indicates the type of tissue and its function in plants? [1]
(a) Parenchyma – Provides mechanical support and protection
(b) Collenchyma – Carries out photosynthesis
(c) Xylem – Transports water and minerals from roots to leaves
(d) Phloem – Provides flexibility and stores food
Answer:
(c) Xylem – Transports water and minerals from roots to leaves
Explanation:
Xylem transports water and minerals from the roots to the leaves and also provides mechanical strength.
![]()
Question 4.
The cell was unable to produce enough energy (ATP). This may be due to malfunction of [1]
(a) ribosomes
(b) mitochondria
(c) Golgi apparatus
(d) endoplasmic reticulum
Answer:
(b) mitochondria
Explanation:
Mitochondria are important cell organelles often called the “powerhouse” of the cell because they are the sites where energy is produced in the form of ATP (Adenosine Triphosphate).
Question 5.
Which of the following is not a component of phloem tissue? ‘ [1]
(a) Sieve tubes
(b) Companion cells
(c) Xylem vessels
(d) Phloem fibres
Answer:
(c) Xylem vessels
Explanation:
Phloem tissue consists of sieve tubes, companion cells, phloem fibres and phloem parenchyma.
Question 6.
Which of the following statements about crop variety improvement are correct? [1]
(i) Use of high-yielding varieties increases production.
(ii) Irrigation helps in providing adequate water to crops.
(iii) Use of chemical fertilisers harms the soil fertility.
(iv) Crop rotation helps in maintaining soil nutrients.
(v) Proper harvesting methods improve crop yield.
(a) (i), (ii), (iii)
(b) (i), (ii), (iv)
(c) (ii), (iii), (v)
(d) (i), (iii), (v)
Answer:
(b) (i), (ii), (iv)
Explanation:
Statement (i), (ii) and (iv) are correct, as High-yielding varieties increases production, irrigation provides necessary water and crop rotation helps maintain soil nutrients.
Question 7.
Which of the following options correctly describes the main function of xylem tissue in plants? [1]
(a) Transport of food from leaves to other parts of the plant.
(b) Transport of water and minerals from roots to leaves.
(c) Photosynthesis and storage of food.
(d) Protection against pathogens and injury.
Answer:
(b) Transport of water and minerals from roots to leaves.
Explanation:
Xylem is a complex permanent tissue in plants responsible for transporting water and dissolved minerals absorbed by the roots to various parts of the plant, mainly the leaves.
The following two questions consist of two statements Assertion (A) and Reason (R). Answer these questions by selecting the appropriate option given below.
(a) Both (A) and (R) are true, and (R) is the correct explanation of (A).
(b) Both (A) and (R) are true, but (R) is not the correct explanation of (A).
(c) (A) is true, but (R) is false.
(d) (A) is false, but (R) is true.
Question 8.
Assertion (A) Squamous epithelium forms the lining of oesophagus and mouth.
Reason (R) Squamous epithelium is thin and allows easy exchange of substances.
Answer:
(a) Both (A) and (R) are true, and (R) is the correct explanation of (A).
Question 9.
Assertion (A) The growth of plants occur in certain specific regions. [1]
Reason (R) Meristematic tissue after differentiation give rise to permanent tissue.
Answer:
(b) Both (A) and (R) are true, but (R) is not the correct explanation of (A).
Explanation:
It can be corrected as
The dividing tissue known as meristematic tissue are only located at specific regions of plant body.
Question 10.
The organisation of membranes and organelles gives the cell the ability to perform functions like energy production, nutrition and waste removal. Comment upon the statement with justification. [2]
Answer:
The cell is the fundamental structural and functional unit of life. Its various membranes and organelles are organised in a specific manner, which allows the cell to perform essential life processes efficiently like mitochondria produces energy, vacuoles store nutrients and help in the removal of wastes. Endoplasmic reticulum and ribosomes are involved in protein synthesis. Cell membrane controls the entry and exit of substances.
Question 11.
Attempt either A or B [2]
A. Identify the cropping pattern in the following cases and state one example for each.
(i) Maize and soybean grown in the same field in alternating rows.
(ii) Wheat grown in one season and groundnut in the next season.
Answer:
(i) The cropping pattern is Intercropping pattern, e.g. maize and soybean. The advantage of intercropping is better utilisation of nutrients as different crops have different nutrient needs.
(ii) The cropping pattern is crop rotation, e.g. Wheat in Rabi season, groundnut in Kharif season. The advantage of cropping pattern is to maintain soil fertility and reduces pest build-up.
A students often get confused between the different types of cropping patterns.
Or
B. Explain why the choice of crops in this practice depends on moisture and irrigation facilities.
Answer:
The choice of crops depends on moisture and irrigation because different crops require different amounts of water. Proper selection ensures good growth, prevents crop failure and allows cultivation of multiple crops in a year.
Question 12.
The cytoplasm is a vital part of the cell, where most metabolic reactions occur, supporting essential functions like energy production and protein synthesis.
If a cell’s cytoplasm is removed, which major cellular function would be immediately affected? Explain briefly. [2]
Answer:
Cytoplasm is a jelly-like substance that fills the cell and holds all the organelles in place. Without cytoplasm, organelles like mitochondria, ribosomes, and endoplasmic reticulum cannot function properly, and the cell would lose its structural and functional integrity.
![]()
Question 13.
Draw the labelled diagram of animal cell and describe the function of any two organelles. [3]
Answer:

The animal cell is a structural and functional unit of an organism.Two important organelles in a cell are
(a) Nucleus It Acts as the control center of the cell. It also contains genetic material (DNA) which directs protein synthesis.
(b) Endoplasmic Reticulum Network of membranes present in the cytoplasm. Rough ER has ribosomes that helps in protein synthesis.
Question 14.
In a biology study, samples of connective tissues from three different body parts were observed under a microscope: [3]
Sample A Made of fluid matrix with cells and plasma.
Sample B Made of dense, parallel collagen fibres with very little ground substance.
Sample C Made of solid matrix containing calcium salts.
(i) Identify the connective tissue in each sample.
(ii) State one function of each connective tissue identified.
Answer:
(i) Sample A is Blood and Sample B is Tendon and Sample C is Bone (skeletal connective tissue).
(ii) Functions of Blood is to transport gases like oxygen, carbon dioxide, nutrients, hormones, and waste products throughout the body. Function of Tendon is to connect muscles to bones and enable movement.
Function of Bone is to provides structural support, protection to internal organs, and helps in movement.
Question 15.
Riya observed the diagram of an animal cell in her science textbook. Help her understand the structure and functions of different cell organelles by answering the questions given below. [4]
Attempt either subpart A or B.
A. Which organelle is known as the powerhouse of the cell? In which type of cell is it found?
Answer:
Mitochondria is known as the powerhouse of the cell and it is found in both plant and animal cells.
Or
B. Which organelle is responsible for the synthesis of proteins? Where is it found in the cell?
Answer:
Ribosome is responsible for protein synthesis.
It can be found floating freely in the cytoplasm or attached to the Rough Endoplasmic Reticulum (RER).
C. Which organelle in the animal cell is known as the control centre? What substance does it contain that carries genetic information?
Answer:
Nucleus is the control centre of the cell. It contains a substance called DNA, which carries genetic information and controls all the activities of the cell.
D. The figure below shows labelled parts of an animal cell. Which of these parts helps in removing waste from the cell?
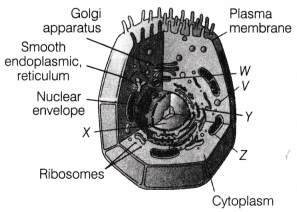
Answer:
The labelled part V is the lysosomes that helps in removing waste from the cell as
It contains enzymes that breakdown waste materials and damaged organelles.
Question 16.
Attempt either A or B. [5]
A. Aman decided to start bee-keeping as an additional source of income.
(i) Where should he place his beehives – near flowering crops like sunflower or in an open area with very few plants? Give a reason for your choice.
(ii) Why is it necessary to handle bees gently and maintain healthy hives? Explain with reason.
Answer:
(i) Aman should place his beehives near flowering crops like sunflower during their flowering season. This is because bees collect nectar from flowers, which they use to produce honey. Placing hives near flowering plants ensures an abundant supply of food for the bees, leading to better honey production.
To obtain maximum marks, students should mention that bees collect nector from flowers for Honey Production.
(ii) Bees should be handled gently and hives kept healthy because rough handling can make bees aggressive, cause injury to them and disturb honey production. Healthy hives prevent diseases in bees and ensure strong colonies, which in turn increases honey yield and quality.
Or
B. Anita grows wheat on two plots of land. On one plot, she uses a combination of fertilisers and irrigation. On the other plot, she relies only on rainwater and does not use any fertilisers.
(i) From which plot will Anita get a better wheat yield? Give reason for you answer.
(ii) Flow does irrigation help in improving crop production?
Answer:
(i) Anita will get a better wheat yield from the plot where she uses fertilisers and irrigation. Fertilisers provide the necessary nutrients for plant growth and irrigation supplies adequate water, both of which support healthy crop development and increase yield.
(ii) Irrigation helps in improving crop production by providing a regular and controlled supply of water to the plants. This ensures that crops do not suffer from drought or water stress, leading to better growth and higher yields even when rainfall is insufficient.
Section – B
Question 17.
Which of the following options correctly represents 1 atomic mass unit (1 amu)?
(a) Mass of hydrogen molecule
(b) 1/12th of mass of C-12 atom
(c) Mass of 0-16 atom
(d) Mass of C-12 atom
Answer:
(b) 1 amu = \(\frac{1}{12}\)th of mass of C-12 atom.
Question 18.
Calculate the volume by volume percentage of a solution of 15 mL of alcohol in 60 mL of water?
(a) 20%
(b) 25%
(c) 30%
(d) 50%
Answer:
(a) 20%
Explanation:
As we know,
Volume by volume percentage of a solution
= \(\frac{\text { Volume of solute }}{\text { Volume of solution }}\) × 100
Volume of solution
Given, volume of alcohol = 15 mL
Volume of water – 60 mL
Volume of solution = 15 + 60 = 75 mL
% \(\frac{V}{V}=\frac{15}{75}\) × 100 =20%
![]()
Question 19.
Four statements about phase changes and heat energy are listed.
I. Condensation releases heat.
II. Vaporisation absorbs heat.
III. Freezing releases heat.
IV. Melting absorbs heat.
Which processes release heat?
(a) I and II
(b) I and III
(c) II and IV
(d) III and IV
Answer:
(b) I and III
Explanation:
Among the given processes, condensation and freezing releases heat while vaporisation and melting absorbs heat.
Question 20.
Unicellular organisms using oxygen dissolved in water because of the phenomenon of
(a) osmosis
(b) diffusion
(c) sublimation
(d) transpiration
Answer:
(b) diffusion
Explanation:
Unicellular organisms use oxygen dissolved in water because of the phenomenon of diffusion.
The spontaneous mixing of the particles of solids or liquids or gases to form homogeneous mixtures is called diffusion.
Question 21.
Tincture of iodine has antiseptic properties. This solution is made by dissolving iodine in which medium? [1]
| Option | Sample | Medium Used to Dissolve Iodine |
| (a) | A. | Iodine in potassium iodide |
| (b) | B. | Iodine in vaseline |
| (c) | C. | Iodine in water |
| (d) | D. | Iodine in alcohol |
Answer:
(d)
Explanation:
Tincture iodine has antiseptic properties. This solution is made by dissolving 2-7% iodine into the alcohol.
Question 22.
A solution contains 16 g of urea in 120 g of the solution. What is the mass percentage of the solution? [11
(a) 14%
(b) 12.4%
(c) 13.3%
(d) 20%
Answer:
(c) 13.3%
Explanation:
Given,
Mass of urea = 16 g
Mass of the solution = 120 g
Mass percentage of the solution
= \(\frac{\text { Mass of urea }}{\text { Mass of the solution }}\) × 100
= \(\frac{16 \mathrm{~g}}{120 \mathrm{~g}}\) × 100
= 133%
To get maximum marks, one should write the correct formula of mass percentage along with all the steps involved in calculation.
Question 23.
Find out the valency of the atoms represented by the Fig. (1) and Fig. (2) given below. [1]

(a) 1 and 2 respectively
(b) 0 and 1 respectively
(c) 1 and 1 respectively
(d) 0 and 2 respectively
Answer:
(b) 0 and 1 respectively
Explanation:
The electronic configuration in fig. (1) is 2,8,8. As, its K, L, and M shells are completely filled, its valency will be zero. It is an inert gas, argon (Ar). The electronic configuration in fig. (2) is 2,7 . As, it has seven electrons in its valence shell, it can gain one electron to complete the octet, so its valency is one. The atom belongs to the element fluorine (F).
The following question consists of two statements -Assertion (A) and Reason (R). Answer the question by selecting the appropriate option given below.
(a) Both (A) and (R) are true and (R) is the correct explanation of (A).
(b) Both (A) and (R) are true, but (R) is not the correct explanation of (A).
(c) (A) is true, but (R) is false.
(d) (A) is false, but (R) is true.
Question 24.
Assertion (A) A mixture of camphor and ammonium chloride cannot be separated by sublimation. [1]
Reason (R) Camphor on heating sublimes while ammonium chloride does not.
Answer:
(c) (A) is true, but (R) is false.
Explanation:
(R) can be corrected as, both camphor and ammonium chloride sublimes on heating.
![]()
Question 25.
Give reasons for the following. [2]
(a) Copper sulphate solution in water does not show Tyndall effect.
(b) Mixture of water and milk shows Tyndall effect.
Answer:
(a) The solution of copper sulphate in water is a true solution. In case of true solution, the solute particles are so small that they can not scatter light falling on them. Hence, copper sulphate solution in water does not show ‘Tyndall effect’.
(b) Mixture of water and milk is a colloid and in colloidal solution, the particles are big enough to scatter light. So, the mixture of water and milk shows “Tyndall effect”.
Question 26.
Attempt either A or B. [3]
A. (a) Identify which of the following pairs are isotopes and which are isobars? Give reasons for your choice.
58A26, 58B28, 79X35, 80Y35
(b) Do isobars also have identical chemical properties like isotopes? State reason.
Answer:
(a) A and B are isobars as they are atoms of different elements having the same mass number.
X and Y are isotopes as they are atoms of same element (same atomic number) having different mass numbers.
(b) No, isobars do not have identical chemical properties because they have different atomic numbers as well as electronic configurations.
Or
B. Given below is the atomic structure of an atom of element fj1 A, according to the Bohr’s model of atom.

(a) What is wrong with this structure of atom?
(b) Draw a correct representation of this atom.
(c) Write the chemical formula of the chloride and sulphate of this element.
Answer:
(a) The element A is Na. It should have three shells K L, and M but here only 2 shells are given. Further, L-shell can not have more than 8 electrons but here 9 electrons are shown. [1]
(b) The correct structure is

(c) As, Na has 1 valence electron, thus it has a valency of 1 and chlorine has a valency of -1.
Hence, the formula of its chloride is A Cl, i.e., NaCl.
Similarly, Na has 1 valence electron, thus it has a valency of +1 and sulphate has a valency of -2. Hence, the formula of its sulphate is Na2SO4.
Question 27.
A student heats ice in a beaker using the setup given below while stirring continuously and recording temperature. [3]

(a) What will the thermometer show when ice starts melting?
(b) Why does the temperature remain constant during melting despite heating?
(c) If the burner is turned off during melting what change would occur in thefmometer reading?
Answer:
(a) The thermometer will show 0°C when ice starts melting, as this is its melting point.
(b) The temperature remains constant because the heat energy is used to break the intermolecular bonds between the ice particles (latent heat of fusion) rather than increasing the temperature.
(c) When the burner is turned off, the temperature drops immediately because the heat supply is cut off.
Question 28.
Rahul was studying the Bohr-Bury scheme to analyse how electrons are distributed in different atoms. He observed four elements (P, Q, R, S) with their electron configurations recorded in a table. To test his understanding, he decided to determine their valency, chemical reactivity, and stability patterns. Based on his observations, Rahul formulated questions to explore these concepts further. Let’s analyse his findings and answer the questions that follow. [4]
| Element | Electron Distribution |
| P | 2, 8, 2 |
| Q | 2, 8, 6 |
| R | 2, 8, 8 |
| S | 2, 5 |
Answer the following questions based on the above information.
A. Which element(s) have a valency of 2?
(a) Only P
(b) P and Q
(c) P and S
Justify your answer.
Answer:
(b) Elements P and Q both can have valency of 2 as in case of element P it can easily lose its two electrons to attain noble gas configuration whereas element Q can gain two electrons to attain the same.
B. The element P (2, 8, 2) reacts with element S (2, 5) to form a compound. Write the chemical formula of the compound formed.
Answer:
The chemical formula of the compound formed is P3S2 (e.g., if P = Magnesium and S = Nitrogen > Mg3N2). This is because P loses
2 electrons while S gains 3 electrons to achieve stability.
Or
Tire element R is chemically inert. Give reason.
Answer:
R is chemically inert because its outermost electron shell is completely filled (2, 8, 8 configuration). This stable octet configuration makes it unreactive like noble gases.
C. Which statement about these elements is correct and why?
(a) Element Q will lose 6 electrons to achieve stability.
(b) Element S has higher electronegativity than element P.
(c) Element R can form covalent bonds easily.
(d) Element P is a non-metal.
Answer:
(b) Element S has higher electronegativity than element P.
Explanation:
Elements has higher electronegativity than element P because S (2, 5) is a non-metal that tends to gain electrons to complete its octet, while P (2, 8, 2) is a metal that tends to lose electrons. Non-metals generally have higher electronegativity than metals.
Question 29.
Attempt either A or B. [5]
A. Answer the following questions.
(a) Classify the following molecules/compounds based on their atomicity.
1. F2
2. NO2
3. N2O
4. C2H6
5. P4
6. H2O2
Answer:
Atomicity of the given molecules/compounds is
1. F2 → Diatomic
2. NO2 → Triatomic
3. N2 O → Triatomic
4. C2H6 → Polyatomic
5. P4 → Tetraatomic
6. H2 O2 → Tetraatomic
(b) Arrange the above molecules/compounds in increasing order of their atomicity.
Answer:
The increasing order of the molecules/compounds according to their atomicity is
F2 < NO2, ≈ N2O < P4 ≈ H2O2, < C2H6
(c) Explain the term atomicity. Give one example each of monoatomic and triatomic molecules not listed above.
Answer:
Atomicity refers to the number of atoms present in one molecule of an element or compound. Helium (He) exists as single atoms (monoatomic) and ozone (03) exists as triatomic.
(d) Why is P4 considered polyatomic despite being a single element?
Answer:
P4 is polyatomic because it exists as a molecule composed of four phosphorus atoms chemically bonded together.
(e) Find the ratio by mass of the combining elements in the following compounds.
C2H6 and H2O2
Answer:
The ratio by mass of the given compounds is
1. Ethane (C2H6)
Carbon: Hydrogen
= (12 × 2): (1 × 6)
= 24 : 6 = 4 : 1
2. Hydrogen peroxide (H2O2)
Hydrogen: Oxygen
= (1 × 2): (16 × 2)
= 2 : 32
= 1 : 16
Or
B. The following ions are given below and answer the question that follows.
Cu2+, Na+, Fe3+, Cl–, SO42-, PO43-
(a) Write molecular formulae of two compounds formed between Na+ and Cl–, Fe3+ and SO42-]
(b) Calculate the molecular mass of copper(II) chloride and sodium phosphate.
[Given, atomic mass of Cu = 63u, Na = 23u, Cl = 35.5u, P = 31u, O = 16u]
(c) Identify which of the above compounds formed will have the highest molecular mass.
(d) Write the correctly balanced formula for iron(III) phosphate showing charge cancellation.
(e) Why can’t Cu2+ combine with PO43- in 1 :1 ratio? Give the correct formula.
Answer:
(a) The molecular formulae of two compounds formed is NaCl (Sodium chloride) and Fe2(SO4)3 (Iron(III) sulphate).
(b) The molecular mass of copper(II) chloride and sodium phosphate is
CuCl2 : 63 + (35.5 × 2) = 134 u
Na3PO4 :(23 × 3) + 31 + (16 × 4) = 164u
Don’t make mistake in calculating molecular mass of the compounds by not multiplying the atoms with their correct atomicity and atomic masses.
(c) Sodium phosphate (Na3PO4)has the highest molecular mass which can be calculated as below.
Molecular mass = 3 × 23u + 31u + 4 × 16u
= 69u + 31u + 64u
= 164u
(d) Formula of iron (III) phosphate is FePO4

Formula is FePO4
(e) Cu2+ and PO4+ can’t combine in 1 :1 because their valencies are +2 and -3. Correct formula is

Formula is Cu3(P04)2.
![]()
Section – C
Question 30.
When an object is floating on the surface of a liquid, the upthrust acting on it is [1]
(i) rqual to the weight of the object.
(ii) less than the weight of the object.
(iii) greater than the weight of the object.
(a) (i)
(b) (ii)
(c) (i) and (ii)
(d) (i), (ii) and (iii)
Answer:
(a) (i)
Explanation:
For an object to float, the upthrust (buoyant force) must balance the weight of the object exactly.
Question 31.
Choose the correct option which explains, why objects fall to the ground? [1]
(a) Earth attracts all objects due to its gravitational force.
(b) Objects fall because they are pushed by air pressure.
(c) Objects fall because they have weight pulling them down.
(d) Objects fall because of magnetic forces from earth.
Answer:
(a) Earth attracts all objects due to its gravitational force.
Explanation:
Earth exerts a gravitational force pulling all objects toward its centre, causing them to fall.
The following question consist of two statements -Assertion (A) and Reason (R). Answer the question by selecting the appropriate option given below.
(a) Both (A) and (R) are true and (R) is the correct explanation of (A).
(b) Both (A) and (R) are true, but (R) is not the correct explanation of (A).
(c) (A) is true, but (R) is false.
(d) (A) is false, but (R) is true.
Question 32.
Assertion (A) Work done by a force can be zero even when the body is in motion. [1]
Reason (R) When force is perpendicular to displacement, the work done is zero.
Answer:
(a) Both (A) and (R) are true, and (R) is the correct explanation of (A).
Explanation:
In circular motion, centripetal force is perpendicular to displacement, hence no work is done.
Question 33.
Velocity-time graph of a 100 g marble rolling on floor is given below. [2]

Calculate
(i) time in which it stops.
(ii) negative acceleration produced in it.
(iii) positive force acting on the marble.
Answer:
From graph,
(i) The velocity reaches zero at t = 30 s tstop = 30 s
(ii) a = \(\frac{(0-40)}{30}\) = -1.33m/s2
(iii) F = ma = \(\frac{100}{1000 x}\) (-1.33) = -0.133 N
|F| = 0.133 N
Time might be in minutes/hours, velocity in m/s or km/h unit mismatch ruins the answer,
Question 34.
Attempt either A or B

A. (i) A body of mass m is raised to a vertical height h through two different paths A and B as shown in the figure. What will be the potential energy of the body in the two cases? Give reason for your answer.
(ii) If two bodies X and Y having equal masses are kept at heights of 2 h and 5 h. Calculate the ratio of their potential energies. (Take, g = 10 m/s2)
Answer:
(i) The work done against gravity in both the cases is mgh. It is independent of the path along which the body is moved and it depends only on the initial and final positions of the body. Flence, potential energy of the body is same in both casesfl]
(ii) Potential energies of bodies X and Y,
PEx = mgh1 and PEy = mgh2
PEx = mg(2h) and PEy = mg(5h)
Comparing the PEx and PEy
\(\frac{\left(\mathrm{PE}_{\mathrm{x}}\right)}{\left(\mathrm{PE}_{\mathrm{y}}\right)}=\frac{m g(2 \mathrm{~h})}{m g(5 \mathrm{~h})}\)
= \(\frac{\left(\mathrm{PE}_x\right)}{\left(\mathrm{PE}_y\right)}=\frac{2}{5}\)
= 2:5
Or
B. A railway track which is discussed has a length of 200 m. A person puts his ear at one end of the rail and suddenly another person hits the other end with a metal hammer, as shown in figure.

(i) State an approximate value for the speed of sound in air.
(ii) Sound travels 6000 m/s in steel. Calculate the time it takes for the sound travel with the rail.
Answer:
(i) The approximate value for the speed of sound in air is 344 m/s. [1]
(ii) Given, distance = 200 m speed = 6000 m/s
Time taken = \(\frac{\text { Distance }}{\text { Speed }}=\frac{200}{6000}\) = 0.03 s
Question 35.
(a) A force P acting on a body A of mass 4 kg produces an acceleration of 6 m/s2. An another force Q action on body B of mass 9 kg produces an acceleration of 4 m/s2. Find the ratio of forces P and Q. [3]

(b) If the two forces X and Y are acting on a body as shown above. Find the resultant acceleration is produced in which direction.
Answer:
(a) From Newton’s second law of motion,
F = ma
Here, P = m1a1 = 4 × 6 = 24 N
Q = m2a2 = 9 × 4 = 36 N
Ratio of forces P and Q will be \(\frac{P}{Q}=\frac{24}{36}=\frac{2}{3}\)
P: Q = 2: 3
(b) Here, mass, m = 15 kg
forces, X =20 N and Y =35 N
Resultant force, F = Y – X = (35 – 20) N
= 15 N and F = ma
a = \(\frac{F}{m}=\frac{15}{15}\) = 1 ms-2
Body will move in left direction with acceleration 1 m/s2.
Question 36.
A stone is dropped from the top of a tower 500 m high into a pond of water at the base of the tower. When is the splash heard at the top? [Given, g = 10 ms-2 and speed of sound = 340 ms-1] [3]
Answer:
Time after which the splash is heard = Time taken by the stone to reach the pond + Time taken by splash sound to reach the top of tower.
For the time taken by the stone to reach the pond.
Here, u =0 [ stone is dropped from rest]
From equation of motion, h = ut + \(\frac{1}{2}\) x g x t2
500 = 5t2
t2 = 100
t = 10s
Time taken by splash sound to reach the top of the tower,
t = \(\frac{\text { Distance }}{\text { Speed }}=\frac{500}{340}\)
Time after which splash is heard = 10 + 1.47 ≈ 11.47 s
Question 37.
Study the diagrams below. [3]

A sheet of paper and a crumpled paper are thrown down from the same height and time is noted.
Answer the following questions.
(a) Will the time taken to reach the bottom container be the same for the sheet of paper and crumpled paper? Why? Explain giving reason.
(b) What will happen in case (II)?
Answer:
(a) Since, the area of a sheet of paper is more than the area of the paper crumpled into a small ball, therefore a sheet of paper will experience a large opposing force due to air than the ball, while falling down. Hence, a sheet of paper falls slower than one that is crumpled into a ball.
(b) Both will take the same time to reach the bottom as there is no air resistance.
Question 38.
A ball is thrown vertically upwards. When it rises, the gravitational force does negative work on decreasing its kinetic energy. As the ball descends, the gravitational force does positive work on it, increasing its kinetic energy. The ball falls back to the point of projection with same velocity and kinetic energy with which it was thrown up. The net work done by the gravitational force on the ball during the round trip is zero because work done by the gravity on displacing a body from one point to another point depends only on the end positions of the body. [4]

A. Answer the following questions.
(i) Why do we say work done against gravity is negative?
(ii) A man is holding a suitcase at his hand at rest. What is work done by him?
Answer:
(i) It is because force and displacement are in opposite directions to each other.
(ii) The work done by him is zero, as displacement is zero.
B. What will be the net work done by the conservative force during the round trip of a body?
Answer:
Work done by the conservative force depends only on initial and final position of the object. During round trip of a body, initial and final position of the body coincides, hence work done by conservative force is zero.
Attempt either C or D
C. (i) When the ball moves vertically upwards, then work done by gravitational force is negative. Why?
(ii) Name the energy of the ball which remains same during round trip of the ball.
Answer:
(i) When the ball moves vertically upwards, then angle between the direction of gravitational force and displacement remains 180°.
Therefore, work done by gravitational force on the ball during upward movement is negative.
(ii) Total mechanical energy (sum of kinetic and potential energy).
Or
D. A ball is thrown vertically upwards with an initial velocity of 20 m/s. Calculate the maximum height reached by the ball. (Assume the acceleration due to gravity is 9.8 m/s2).
Answer:
Using the equation of motion,
v2 = u2 + 2 as
At maximum height, the final velocity v = 0,
initial velocity u =20 m/s,
acceleration a = -98 m /s2 and s is the maximum height.
0 =(20)2 + 2(-9.8)s
0 = 400 – 19.6 s
s = \(\frac{400}{196}\)s = 20.41 m
Question 39.
Attempt either A or B [5]

A. (a) What type of motion is represented by PQ, OP, QR?
(b) Calculate the retardation of body?
(c) Find the distance travelled by the body from P to Q.
Answer:
(a) PQ represents straight line graph between speed and time which is parallel to the time axis, so PQ represents uniform speed. There is no acceleration from P to Q.
OP is a straight line graph between speed and time and it is sloping upwards from O to P. Therefore, the graph OP represents uniform acceleration.
QR is a straight line graph between speed and time which is sloping downwards from Q to R. Therefore, QR represents uniform retardation or negative acceleration.
(b) The slope of line graph QR represents the retardation of the body.
So, retardation = slope of line QR = \(\frac{Q T}{T R}\)
We have QT = 6 m/s,
TR = 16 – 10 = 6 s
Retardation =6 ms-1 / 6 s = 1 m/s2
(c) Distance travelled from Pto Q =Area under the line PQ and the time axis = Area of rectangle
Here, SP = 6 m/’s
and ST =10 – 4 = 6 s
Distance travelled from P to Q = 6 × 6 = 36m
Or
B. A vehicle of 1 tonne travelling with a speed of 60 ms notices a cow on the road 9 m ahead and applies brakes. It stops just in front of the cow.
(a) Find out the KE of the vehicle before applying brakes and also calculate the retarding force provided by the brakes.
(b) How much time did it take to stop after the brakes were applied?
(c) What is the work done by the braking force?
Answer:
Given, mass of the vehicle, m = 1 tonne = 1000 kg
initial speed, u = 60 m/s
distance between vehicle and the cow, s = 9 m
final velocity, v = 0
(a) KE of vehicle before applying brakes is given by
= \(\frac{1}{2}\) × mu
= \(\frac{1}{2}\) × 1000 × 60 × 60
= 1800000 J
Now, calculate retarding force from the third equation of motion,
v2 – u2 = 2 as
(0)2 – (60)2 = 2 × a × 9
a = -200 m/s2
So retarding force provided by the brakes = ma = 1000 × (-200)
= -200000 N
![]()
(b) Now, again from the second equation of motion,
s = ut + \(\frac{1}{2}\)at2
9 = 60t + \(\frac{1}{2}\) × (-200)t2
9 = 60t – 100t2
(10t -3)2 =0
10t – 3 =0
f = \(\frac{3}{10}\) = 03 s
(c) So, work done by the braking force is given by
= Fs = -200000 × 9
=-1800000 J
The post CBSE Sample Papers for Class 9 Science Set 8 with Solutions appeared first on Learn CBSE.
📚 NCsolve - Your Global Education Partner 🌍
Empowering Students with AI-Driven Learning Solutions
Welcome to NCsolve — your trusted educational platform designed to support students worldwide. Whether you're preparing for Class 10, Class 11, or Class 12, NCsolve offers a wide range of learning resources powered by AI Education.
Our platform is committed to providing detailed solutions, effective study techniques, and reliable content to help you achieve academic success. With our AI-driven tools, you can now access personalized study guides, practice tests, and interactive learning experiences from anywhere in the world.
🔎 Why Choose NCsolve?
At NCsolve, we believe in smart learning. Our platform offers:
- ✅ AI-powered solutions for faster and accurate learning.
- ✅ Step-by-step NCERT Solutions for all subjects.
- ✅ Access to Sample Papers and Previous Year Questions.
- ✅ Detailed explanations to strengthen your concepts.
- ✅ Regular updates on exams, syllabus changes, and study tips.
- ✅ Support for students worldwide with multi-language content.
🌐 Explore Our Websites:
🔹 ncsolve.blogspot.com
🔹 ncsolve-global.blogspot.com
🔹 edu-ai.blogspot.com
📲 Connect With Us:
👍 Facebook: NCsolve
📧 Email: ncsolve@yopmail.com

😇 WHAT'S YOUR DOUBT DEAR ☕️
🌎 YOU'RE BEST 🏆